آمفتامین استخلافی
آمفتامینهای استخلافی دسته ای از ترکیبات شیمیایی هستند که ساختار هسته اصلی آن بر اساس ساختار آمفتامین بنا شدهاست.[۱] این ترکیبات شامل تمام ترکیبات مشتق شده از آمفتامین به وسیله جابجایی یا جانشینی یک یا چند اتم هیدروژن در ساختار هسته آمفتامین با یک استخلاف است.[۲][۳][۴][۵] ترکیبات موجود در این دسته انواع مختلفی از زیردستههای دارویی را شامل میشوند، از جمله محرکها، empathogenها، توهمزاها و غیره.[۶] نمونههای آمفتامین استخلافی عبارت است از: آمفتامین (ترکیب هسته سازنده)،[۷][۸]متآمفتامین،[۹] افدرین،[۱۰] کاتینون،[۱۱] فنترمین،[۱۲] مفنترمین،[۱۳] بوپروپیون،[۱۴] متوکسی فنامین،[۱۵] سلژیلین،[۱۶] آمفپرامون،[۱۷] پیرووالرون،[۱۸] MDMA (اکستازی) و DOM (STP).
| آمفتامین استخلافی | |
|---|---|
| کلاس دارویی | |
 ساختار آمفتامین | |
| شناسههای دستهبندی | |
| طبقهبندی شیمیایی | مشتقات استخلافی آمفتامین |
| در ویکیداده | |
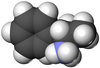 |

|
| ال-آمفتامین | دی-آمفتامین |
فهرست برخی از آمفتامینهای استخلافی ویرایش
| نام ژنریک یا غیررسمی | نام شیمیایی | # تعداد استخلافها |
|---|---|---|
| آمفتامین | α-Methyl-phenethylamine | ۰ |
| متآمفتامین | N-Methylamphetamine | ۱ |
| Ethylamphetamine | N-Ethylamphetamine | ۱ |
| پروپیل آمفتامین | N-Propylamphetamine | ۱ |
| Isopropylamphetamine | N-iso-Propylamphetamine | ۱ |
| Phentermine | α-Methylamphetamine | ۱ |
| فنیل پروپانول آمین (PPA) | β-Hydroxyamphetamine, (1R,2S)- | ۱ |
| کاتین (شیمی) | β-Hydroxyamphetamine, (1S,2S)- | ۱ |
| کاتینون | β-Ketoamphetamine | ۱ |
| اورتتامین | 2-Methylamphetamine | ۱ |
| 2-Fluoroamphetamine (2-FA) | 2-Fluoroamphetamine | ۱ |
| 3-Methylamphetamine (3-MA) | 3-Methylamphetamine | ۱ |
| 2-Phenyl-3-aminobutane | 2-Phenyl-3-aminobutane | ۱ |
| 3-Fluoroamphetamine (3-FA) | 3-Fluoroamphetamine | ۱ |
| نورفنفلورآمین | 3-Trifluoromethylamphetamine | ۱ |
| 4-Methylamphetamine (4-MA) | 4-Methylamphetamine | ۱ |
| para-Methoxyamphetamine (PMA) | 4-Methoxyamphetamine | ۱ |
| para-Ethoxyamphetamine | 4-Ethoxyamphetamine | ۱ |
| 4-Methylthioamphetamine (4-MTA) | 4-Methylthioamphetamine | ۱ |
| نورفولدرین (α-Me-TRA) | 4-Hydroxyamphetamine | ۱ |
| para-Bromoamphetamine (PBA, 4-BA) | 4-Bromoamphetamine | ۱ |
| para-Chloroamphetamine (PCA, 4-CA) | 4-Chloroamphetamine | ۱ |
| para-Fluoroamphetamine (PFA, 4-FA, 4-FMP) | 4-Fluoroamphetamine | ۱ |
| para-Iodoamphetamine (PIA, 4-IA) | 4-Iodoamphetamine | ۱ |
| Clobenzorex | N-(2-chlorobenzyl)-1-phenylpropan-2-amine | ۱ |
| Dimethylamphetamine | N,N-Dimethylamphetamine | ۲ |
| Benzphetamine | N-Benzyl-N-methylamphetamine | ۲ |
| D-Deprenyl | N-Methyl-N-propargylamphetamine, (S)- | ۲ |
| سلژیلین | N-Methyl-N-propargylamphetamine, (R)- | ۲ |
| مفنترمین | N-Methyl-α-methylamphetamine | ۲ |
| Phenpentermine | α,β-Dimethylamphetamine | ۲ |
| افدرین | β-Hydroxy-N-methylamphetamine, (1R,2S)- | ۲ |
| سودوافدرین (PSE) | β-Hydroxy-N-methylamphetamine, (1S,2S)- | ۲ |
| Methcathinone | β-Keto-N-methylamphetamine | ۲ |
| Ethcathinone | β-Keto-N-ethylamphetamine | ۲ |
| کلورترمین | 2-Chloro-α-methylamphetamine | ۲ |
| Methoxymethylamphetamine (MMA) | 3-Methoxy-4-methylamphetamine | ۲ |
| فنفلورامین | 3-Trifluoromethyl-N-ethylamphetamine | ۲ |
| Dexfenfluramine | 3-Trifluoromethyl-N-ethylamphetamine, (S)- | ۲ |
| 4-Methylmethamphetamine (4-MMA) | 4-Methyl-N-methylamphetamine | ۲ |
| para-Methoxymethamphetamine (PMMA) | 4-Methoxy-N-methylamphetamine | ۲ |
| para-Methoxyethylamphetamine (PMEA) | 4-Methoxy-N-ethylamphetamine | ۲ |
| فولدرین | 4-Hydroxy-N-methylamphetamine | ۲ |
| Chlorphentermine | 4-Chloro-α-methylamphetamine | ۲ |
| para-Fluoromethamphetamine (PFMA, 4-FMA) | 4-Fluoro-N-methylamphetamine | ۲ |
| زیلوپروپامین | 3,4-Dimethylamphetamine | ۲ |
| α-Methyldopamine (α-Me-DA) | 3,4-Dihydroxyamphetamine | ۲ |
| 3,4-Methylenedioxyamphetamine (MDA) | 3,4-Methylenedioxyamphetamine | ۲ |
| Dimethoxyamphetamine (DMA) | X,X-Dimethoxyamphetamine | ۲ |
| 6-APB | 6-(2-aminopropyl)benzofuran | ۲ |
| کوربادرین (α-Me-NE) | β,3,4-Trihydroxyamphetamine, (R)- | ۳ |
| Oxilofrine | β,4-Dihydroxy-N-methylamphetamine | ۳ |
| Aleph | 2,5-dimethoxy-4-methylthioamphetamine | ۳ |
| دیاوبی (روانگردان) (DOB) | 2,5-Dimethoxy-4-bromoamphetamine | ۳ |
| Dimethoxychloroamphetamine (DOC) | 2,5-Dimethoxy-4-chloroamphetamine | ۳ |
| دیاوئیاف (روانگردان) (DOEF) | 2,5-Dimethoxy-4-fluoroethylamphetamine | ۳ |
| Dimethoxyethylamphetamine (DOET) | 2,5-Dimethoxy-4-ethylamphetamine | ۳ |
| Dimethoxyfluoroamphetamine (DOF) | 2,5-Dimethoxy-4-fluoroamphetamine | ۳ |
| دیاوآی (روانگردان) (DOI) | 2,5-Dimethoxy-4-iodoamphetamine | ۳ |
| دیاوام (روانگردان) (DOM) | 2,5-Dimethoxy-4-methylamphetamine | ۳ |
| دیاوان (روانگردان) (DON) | 2,5-Dimethoxy-4-nitroamphetamine | ۳ |
| Dimethoxypropylamphetamine (DOPR) | 2,5-Dimethoxy-4-propylamphetamine | ۳ |
| Dimethoxytrifluoromethylamphetamine (DOTFM) | 2,5-Dimethoxy-4-trifluoromethylamphetamine | ۳ |
| Methylenedioxymethamphetamine (اکستازی) | 3,4-Methylenedioxy-N-methylamphetamine | ۳ |
| Methylenedioxyethylamphetamine (MDEA) | 3,4-Methylenedioxy-N-ethylamphetamine | ۳ |
| Methylenedioxyhydroxyamphetamine (MDOH) | 3,4-Methylenedioxy-N-hydroxyamphetamine | ۳ |
| 2-Methyl-MDA | 3,4-Methylenedioxy-2-methylamphetamine | ۳ |
| 5-Methyl-MDA | 4,5-Methylenedioxy-3-methylamphetamine | ۳ |
| Methoxymethylenedioxyamphetamine (MMDA) | 3-Methoxy-4,5-methylenedioxyamphetamine | ۳ |
| Trimethoxyamphetamine (TMA) | X,X,X-Trimethoxyamphetamine | ۳ |
| Dimethylcathinone | β-Keto-N,N-dimethylamphetamine | ۳ |
| Diethylcathinone | β-Keto-N,N-diethylamphetamine | ۳ |
| بوپروپیون | β-Keto-3-chloro-N-tert-butylamphetamine | ۳ |
| مفدرون (4-MMC) | β-Keto-4-methyl-N-methylamphetamine | ۳ |
| Methedrone (PMMC) | β-Keto-4-methoxy-N-methylamphetamine | ۳ |
| Brephedrone (4-BMC) | β-Keto-4-bromo-N-methylamphetamine | ۳ |
| Flephedrone (4-FMC) | β-Keto-4-fluoro-N-methylamphetamine | ۳ |
منابع ویرایش
- ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- ↑ Lillsunde P, Korte T (March 1991). "Determination of ring- and N-substituted amphetamines as heptafluorobutyryl derivatives". Forensic Sci. Int. 49 (2): 205–213. doi:10.1016/0379-0738(91)90081-s. PMID 1855720.
- ↑ Custodio, Raly James Perez; Botanas, Chrislean Jun; Yoon, Seong Shoon; Peña, June Bryan de la; Peña, Irene Joy dela; Kim, Mikyung; Woo, Taeseon; Seo, Joung-Wook; Jang, Choon-Gon; Kwon, Yong Ho; Kim, Nam Yong (2017-11-01). "Evaluation of the Abuse Potential of Novel Amphetamine Derivatives with Modifications on the Amine (NBNA) and Phenyl (EDA, PMEA, 2-APN) Sites". Biomolecules & Therapeutics (به انگلیسی). 25 (6): 578–585. doi:10.4062/biomolther.2017.141. ISSN 2005-4483. PMC 5685426. PMID 29081089.
- ↑ Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39).
- ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ↑ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ (2012). "Biosynthesis of amphetamine analogs in plants". Trends Plant Sci. 17 (7): 404–412. doi:10.1016/j.tplants.2012.03.004. PMID 22502775.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). Countless variation in functional group substitutions has yielded a collection of synthetic drugs with diverse pharmacological properties as stimulants, empathogens and hallucinogens [3]. ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. The stereochemistry at the α-carbon is often a key determinant of pharmacological activity, with (S)-enantiomers being more potent. For example, (S)-amphetamine, commonly known as d-amphetamine or dextroamphetamine, displays five times greater psychostimulant activity compared with its (R)-isomer [78]. Most such molecules are produced exclusively through chemical syntheses and many are prescribed widely in modern medicine. For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall and Dexedrine, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines.
کتابشناسی ویرایش
- Ghodse, Hamid (2002). Drugs and Addictive Behaviour. A Guide to Treatment. 3rd Edition. Cambridge University Press. p. 501. ISBN 978-0-511-05844-8.
- Glennon, Richard A. (2008). "Neurobiology of Hallucinogens". The American Psychiatric Publishing textbook of substance abuse treatment. American Psychiatric Publishing. ISBN 978-1-58562-276-4.
- Goldfrank, Lewis R. & Flomenbaum, Neal (2006). Goldfrank's Toxicologic Emergencies, 8th Edition. McGraw Hill. ISBN 978-0-07-147914-1.
- Katzung, Bertram G. (2009). Basic & clinical pharmacology. 11th edition. McGraw-Hill Medical. ISBN 978-0-07-160405-5.[پیوند مرده]
- Ledgard, Jared (2007). A Laboratory History of Narcotics. Volume 1. Amphetamines and Derivatives. Jared Ledgard. pp. 268. ISBN 978-0-615-15694-1.
- Schatzberg, Alan F. & Nemeroff, Charles B. (2009). The American Psychiatric Publishing Textbook of Psychopharmacology. The American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- Snow, Otto (2002). Amphetamine syntheses. Thoth Press. ISBN 978-0-9663128-3-6.
- Veselovskaya NV, Kovalenko AE (2000). Drugs. Properties, effects, pharmacokinetics, metabolism. MA: Triada-X. ISBN 978-5-94497-029-9.
پیوند به بیرون ویرایش
- پروندههای رسانهای مربوط به Substituted amphetamines در ویکیانبار